Impressive Info About How To Tell If A Compound Is Soluble In Water
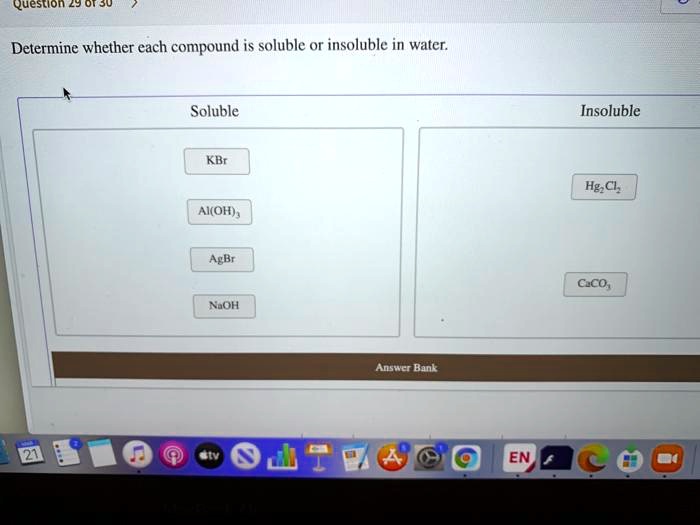
Classify each compound as soluble or insoluble.
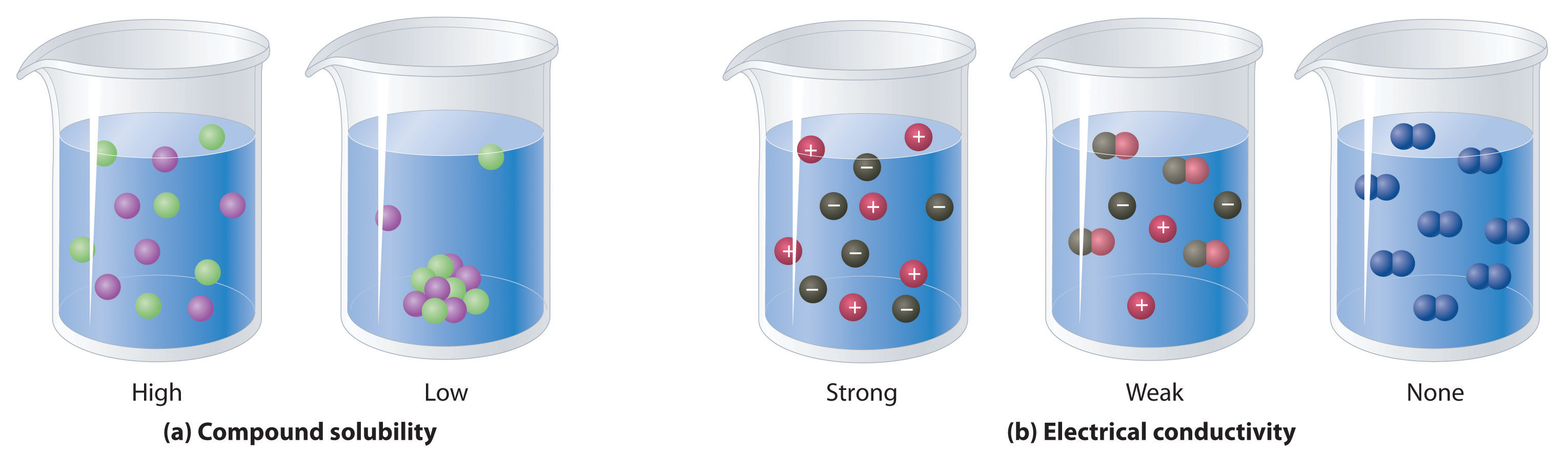
How to tell if a compound is soluble in water. A compound that is soluble in water forms an aqueous solution. This force tends to bring ions into solution. This solution can accept more solute.
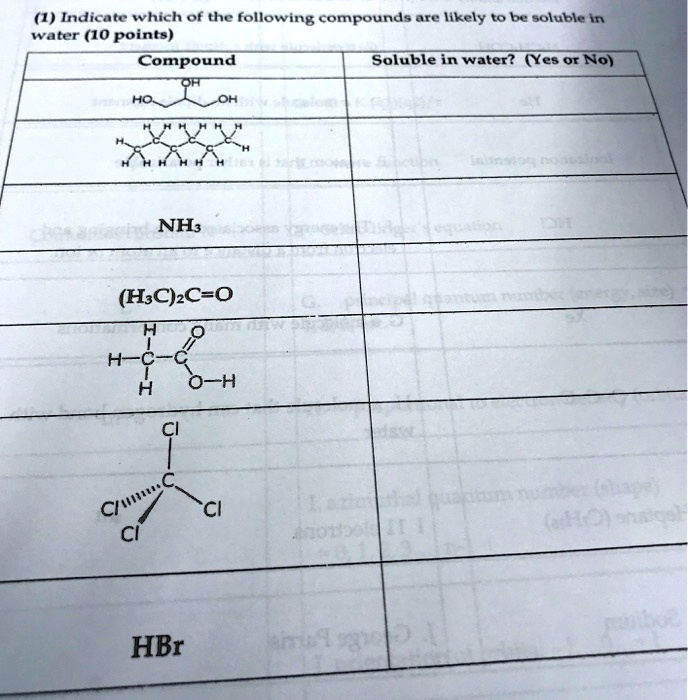
1) if the solution has less solute than the maximum amount that it is able to dissolve (its. Use the solubility rules to predict if a compound is soluble, insoluble, or slightly soluble when some substances are dissolved in water, they undergo either a. Organic compounds tend to dissolve well in solvents that have similar properties to themselves.
This page discusses the solubility of compounds in. While many compounds are partially or mostly insoluble, there is no substance. Force of attraction between h2o molecules and the ions of the solid.
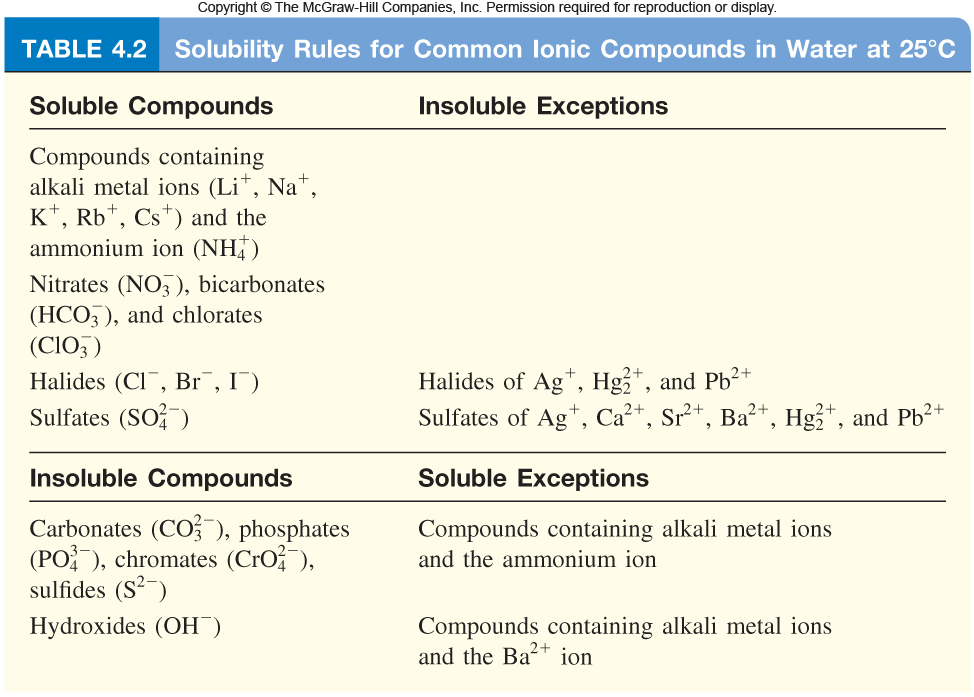
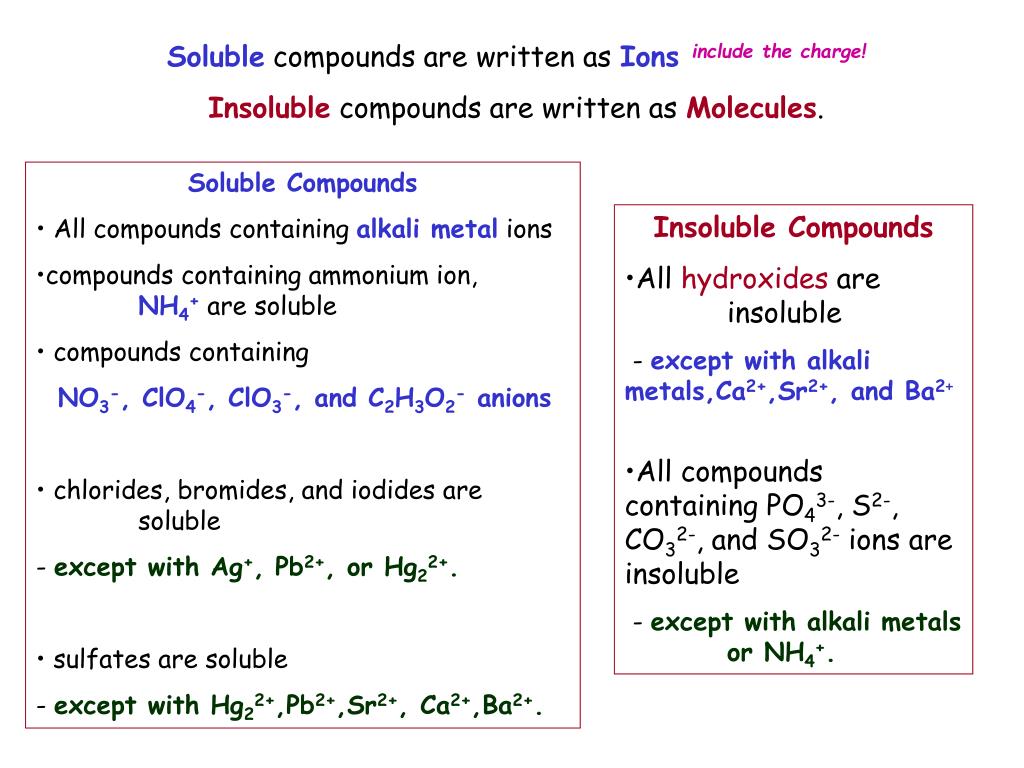
Exceptions to this rule are rare. Identify the compound whose solubility you want to check. Use the solubility rules to determine whether a compound will dissolve or precipitate in water.
Salts containing the ammonium ion (nh 4+) are. How to use solubility rules. A solute is considered insoluble when they are unable to dissolve at a ratio greater than 10000:1.
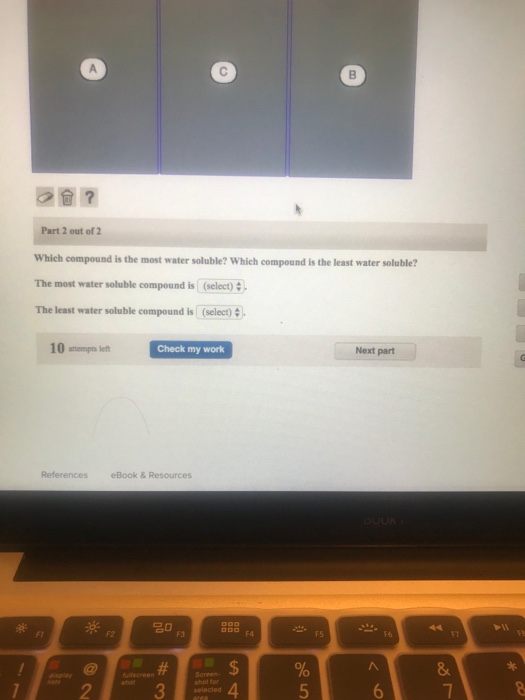
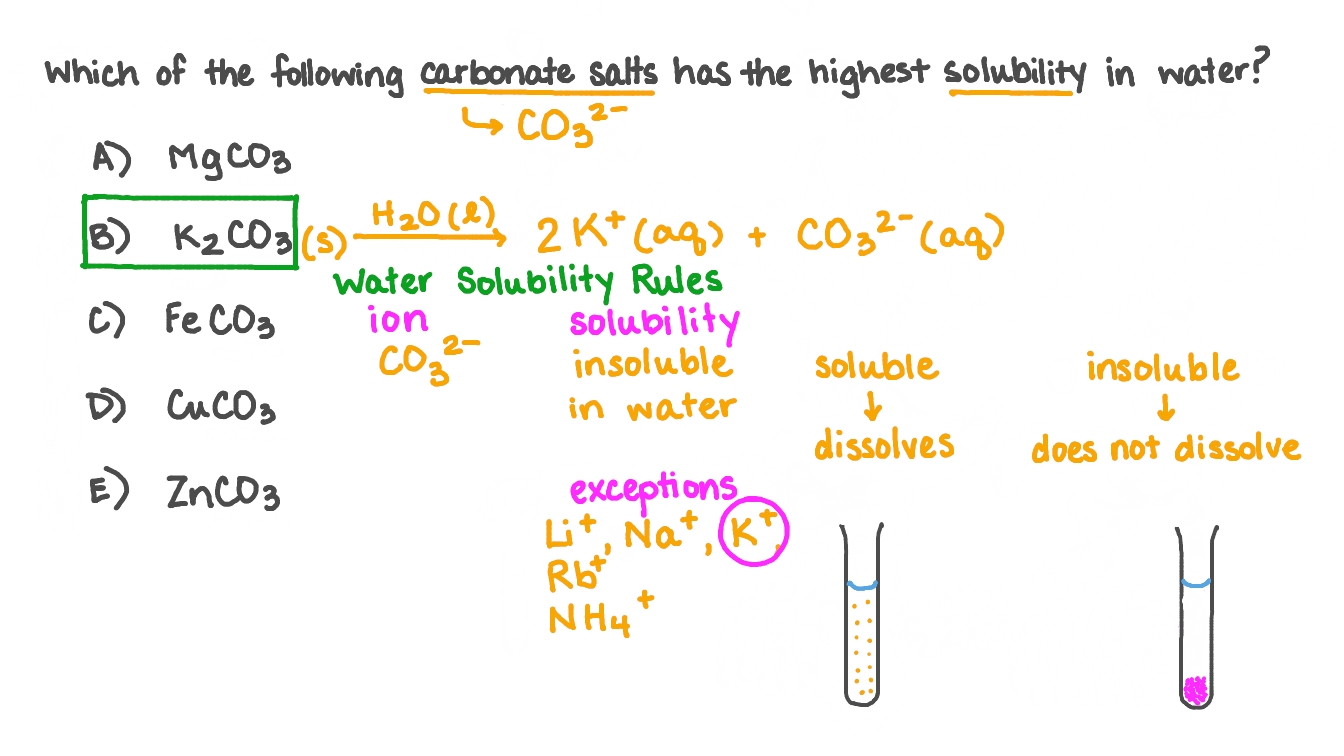
Salts containing group i elements are soluble (li +, na + , k +, cs +, rb + ). Identify the compound whose solubility you want to check. Predict which of the following compounds will be most soluble in water:
Sr 3 (po 4) 2; If this is the predominant factor, then the. Pressure and temperature affect solubility.
If organic compounds molecular mass is small, they have a higher possibility to dissolve in water. Depending on the solubility of a solute, there are three possible results: How to use solubility rules.
Nitrates are soluble in water with no. Would you expect the following molecule to. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well as all compounds containing the alkali.
The opposite is a dilute solution; Hint the water, being polar in nature, dissolves the polar substances in it i.e., we can say that like dissolves like. In the previous post, we talked about the intermolecular interactions and their correlation with the physical properties of.

![[Solved] Which of these functional groups is soluble in 9to5Science](https://i.stack.imgur.com/d213J.jpg)